is sand water homogeneous or heterogeneous
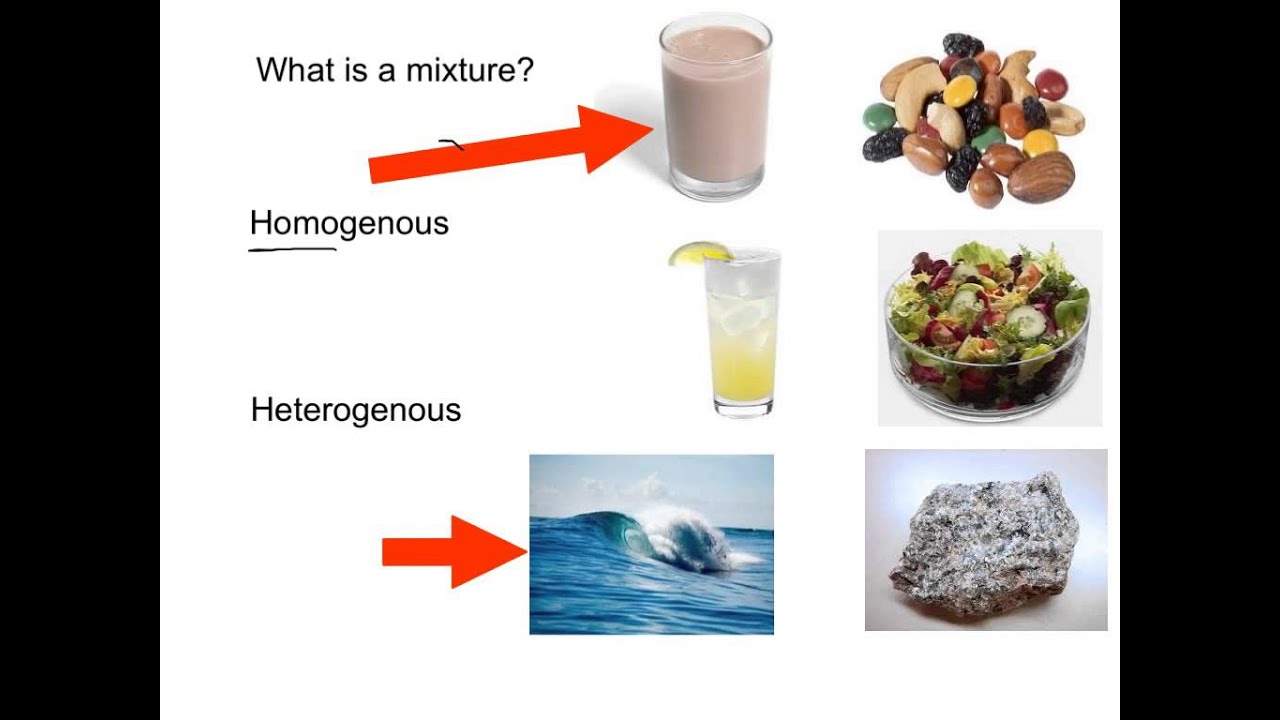
For instance a mixture of water and sand suspension of sulfur in water and granite are heterogeneous mixtures. An example of a homogeneous mixture would be something like lemonade.

Let S Do An Experiment Homogeneous And Heterogeneous Mixtures Heterogeneous Mixture 5th Grade Science Physical Changes Experiments
B A commercial sports drink is a homogeneous mixture because its composition is uniform throughout.

. Heterogeneous mixtures include multi-phase substances different states of matter such as sand and water solid and liquid or carbonated drinks gas and liquid. Over 13 orders of magnitude. You may get different numbers of chocolate chips with each bite.
A mixture in which constituents are distributed uniformly such as salt in water is called homogeneous whereas a mixture whose constituents are clearly separate from one another such as sand in water it is called heterogeneous. Miocene alluvial fan sediments in Southern California. A heterogeneous mixture is a mixture with a non-uniform composition.
Black coffee homogeneous 15. For example bitumen is a homogeneous mixture that is a component of asphalt a heterogeneous mixture. Sugar water homogeneous heterogeneous.
It can be separated out physically homo means the same hetero means different. In chemistry the difference between a homogeneous and heterogeneous mixture is a bit more complicated. Techniques to separate mixtures.
Hence when light is passed through such solutions. Consider how homogenized milk is created. A tossed salad is a heterogeneous mixture.
Heterogeneous photocatalysis is a discipline which includes a large variety of reactions. Dirt and sand are heterogeneous mixtures. Its a homogenous mixture of oxygen nitrogen argon carbon.
The prefixes homo- indicate sameness. Rubbing alcohol homogeneous 4. A heterogeneous mixture consists of.
These are mixtures that are uniform throughout their composition. Sand oil and water and chicken noodle soup are examples of heterogeneous mixtures. Some homogeneous mixtures are components of heterogeneous mixtures.
Homogeneous and heterogeneous are. For example pure water boils at 100 degrees C HOMOGENEOUS MIXTURES. Some solutions are heterogeneous in nature and they are termed as suspension.
A chocolate chip cookie is a heterogeneous mixture. Mixtures of sand and water. Mild or total oxidations dehydrogenation hydrogen transfer 18 O 2 16 O 2 and deuterium-alkane isotopic exchange metal deposition water detoxification gaseous pollutant removal etc.
This makes it much easier to separate these mixtures. Its possible for a mixture to start out as heterogeneous and then become homogeneous as one substance dissolves. Full fat milk heterogeneous 5.
Some mixtures appear homogeneous from a distance but are heterogeneous upon closer inspection. An example would be adding sugar to water. In a mixture all the different parts retain their original properties.
Aerated drinks Salt-water or Sugar water mixtures fruit juices are some examples for solutions. Examples of homogeneous mixtures include air saline solution most alloys and bitumen. It cant be separated out physically.
Elements and compounds are types of pure substances. Pure substances and mixtures. Homogeneous mixture Heterogeneous mixture.
For example water and oilwater and sand. A homogeneous solution tends to be identical no matter how you sample it. Examples of Homogeneous and Heterogeneous Mixtures.
Unlike homogeneous mixtures in heterogeneous mixtures it is very easy to identify even with the naked eye what are the different components that make them up. The small splash of water is full of bits of plant matter and fun little creatures that live in the water like amoebas paramecia and Euglena. Mixtures of gold powder and silver powder.
A mixture of alcohol and water. If you look closely at sand from a beach you can see the different components including shells coral sand and organic matter. The opposite of heterogeneous mixtures is homogeneous mixtures.
Beach sand heterogeneous 6. Telling Homogeneous and Heterogeneous Mixtures Apart. Another example is the air we breathe.
Even a mixture of oil and water is heterogeneous because the density of water and oil is different which prevents uniform distribution in the mixture. Pure air homogeneous 7. Other examples of homogeneous mixtures include air maple syrup gasoline and a solution of salt in water.
Once mixed you cant easily separate the lemon juice from the water. A solution of oil and water water and chalk powder and solution of water and sand etc. A solution is a special type of mixture that is homogeneous where you cannot tell the difference between the components.
Aluminum foil homogeneous 14. A homogeneous mixture is a mixture of two or more chemical substances elements or compounds where the. Thus we can separate the components in that mixture depending on the property variation.
Corn syrup homogeneous 12. Mixtures of sand and iron filings. Examples of heterogeneous mixtures.
A homogeneous mixture has the same uniform appearance and composition throughout. There are patches of dense liquid water falling down through the less dense air. Examples of heterogeneous mixtures include sand oil and water and chicken noodle soup.
When Identifying Homogeneous and. Homogeneous mixtures are sources of water saline solution some alloys and bitumen. The homogenization process is responsible for giving rise to a homogenous mixture from a heterogeneous mixture.
Eg water and oil water and sand. Examples include soil blood and sand. There are 2 types of matter.
Through combining two or more substances a mixture is produced. A solution is also a special type of mixture that cannot be. Sand 10-7 to 10-2 Silt 10-9 to 10-5 Clay Shale 10-12 to 10-9 Karst limestone 10-5 to 10-1 Sandstone 10-10 to 10-5 Igneous Metamorphic rocks 10-13 to 10-10 unfractured Use values in your text and cite them.
Unlike homogeneous mixtures in these it is very easy to identify even to the naked eye which are the different components that make them up. A Oil and vinegar salad dressing is a heterogeneous mixture because its composition is not uniform throughout. Classify the following as an element compound homogeneous mixture or heterogeneous mixture.
Examples of heterogeneous mixtures would be ice cubes before they melt in soda cereal in milk various toppings on a pizza toppings in frozen yogurt a box of assorted nuts. Well a mixture is made up of two or more kinds of matter but sometimes you can still see the different components like sand and water. Examples of homogeneous mixtures include.
Mainly a mixture of debris flow and channel sheetflood. Sand mixed with water mud dust in the air and granite can all be referred to as suspensions. 20 Examples of Heterogeneous Mixtures.
Its an obviously heterogeneous mixture because there are two states or phases of matter. Concrete is a heterogeneous mixture of cement gravel sand and water. Oil and Water Explore the interactions that cause water and oil to separate from a mixture.
If you look at some dirt or sand under a microscope you will be able to see different pieces of material that make up the sample. In addition uniform mixture is another term for homogenous mixture. Homogeneous and heterogeneous mixtures.
In these mixtures the properties are not uniform throughout. Mostly the difference between the two types of mixtures is a matter of scale. An example or a heterogeneous mixture is the atmosphere on a rainy day.
Helium and air. Have you ever looked at a drop of pond water under a microscope. Pure water corn oil blood plasma steel alloys.
Sand may appear homogeneous from a distance yet when you magnify it it is heterogeneous. Italian salad dressing heterogeneous 11. Mixtures can be either homogeneous or heterogeneous.
8 Natural Variation of K Its huge. Chunky spaghetti sauce 8. What do all heterogeneous mixtures have in common.
Eg wine beer gelatin water and alcohol. The rainy atmosphere is a mixture of the air which is a gas with liquid rain droplets. Many homogeneous mixtures are commonly referred to as solutions.
Such suspended particles can be seen quite clearly in the solution. The sugar crystals are solids but when they dissolve the sugar is in. OR classify as a mixture or pure substance.
A mixture of sodium chloride and sand. This makes it much easier to separate these mixes at the same time. A sandwich is a heterogeneous mixture.

Pin By Bean Er On My Cheat Sheeeeets Pure Products Physical And Chemical Properties Heterogeneous Mixture

Physical Science Lessons 5th Grade Science Middle School Science Experiments

How To Identify Heterogeneous Homogeneous Mixtures Food Snacks Good Carbs

Heterogeneous And Homogeneous Mixtures Physical Science Matter Winter Holiday Christmas Christmas Science Middle School Science Activities Winter Holidays

Selina Concise Chemistry Class 6 Icse Solutions Chapter 5 Pure Substances And Mixtures Separation Of Mixtures Ncert Chemistry Class Chemistry Science Notes

Selina Concise Chemistry Class 8 Icse Solutions Chapter 3 Elements Compounds And Mixtures Ncert Books Ncertbooks Chemistry Class Chemistry Matter Science

Grade 5 Science Lesson 8 Matter Mixture And Solution Primary Science Science Lessons Science Primary Science

9th Class Science Notes In English Chapter 2 Is Matter Around Us Pure Gyan Study Point Science Notes Matter Worksheets Matter Science

Pin By Becky Mitchell On Homeschool Heterogeneous Mixture Physics Mixtures

Heterogeneous Homogeneous Mixture Card Sort For Matter In Chemistry Homogeneous Mixture Heterogeneous Mixture Sorting Cards

Experimental Techniques Lab Equipment Worksheets Teaching Resources Compounds And Mixtures Elements Compounds And Mixtures Lab Equipment

Pure Substances And Mixtures Chemistry For Kids Mocomi Chemistry For Kids Pure Products Learning Science

Selina Concise Chemistry Class 6 Icse Solutions Chapter 5 Pure Substances And Mixtures Separation Of Mixtures L Chemistry Class Chemistry Teaching Chemistry

Pin By Divi Goyal On Pictures Science Notes Heterogeneous Mixture Chapter Summary

Mixtures And Pure Substances Heterogeneous Mixture Pure Products Mixtures

C20 Unit 1 2 Classification Of Matter Types Of Mixtures Solution Examples Heterogeneous Mixture


0 Response to "is sand water homogeneous or heterogeneous"
Post a Comment