is zostavax a live vaccine
A live attenuated zoster vaccine ZOSTAVAX Merck Co Inc was approved by the Korea Ministry of Food and Drug Safety in 2009. This is multi-center open-label single-arm study performed with.
Zostavax Full Prescribing Information Dosage Side Effects Mims Thailand
Women should not become pregnant for at least 3 months after receiving this vaccine.
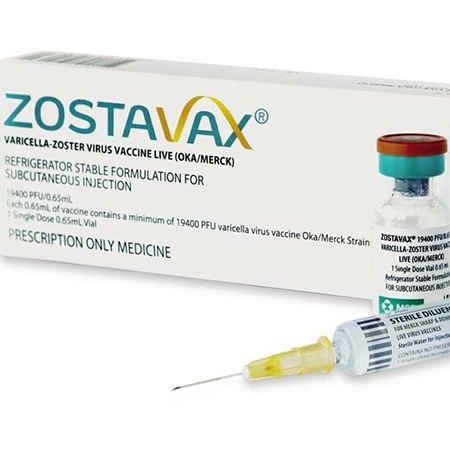
. Zostavax Shingles Vaccine Description. Its similar but not identical to the chickenpox vaccine. The vaccine recommended for most people is a live vaccine called Zostavax.
However due to the. Subcutaneous powder for suspension. If you are a breast-feeding mother and are.
Zoster vaccine is registered in the EU for use in adults aged or50 years for the prevention of herpes zoster and herpes zoster-related postherpetic neuralgia. ZOSTAVAX is used to vaccinate individuals 50 years of age or older. Herpes zoster is caused by the same virus varicella that causes chickenpox in children.
They will be offered a non-live vaccine called Shingrix. Federal government websites often end in gov or mil. However the immunogenicity and safety of the vaccine has not been assessed in Korean population.
Zostavax is a live attenuated varicella zoster virus VZV vaccine developed specifically for the prevention of HZ and PHN in individuals aged 50 years. Zostavax is a live vaccine and should not be given to individuals who have a weakened immune system caused by treatments such as radiation chemotherapy or other. Mercks Zostavax is a live attenuated varicella-zoster virus weakened chickenpox virus vaccine.
Is a live attenuated varicella zoster virus vaccine. Yes Shingrix is an inactive vaccine so you can administer it with other inactive or live vaccines. The vaccine should be administered immediately after reconstitution to minimize loss of potency.
Shingrix recombinant zoster vaccine is the preferred vaccine over Zostavax zoster vaccine live a shingles vaccine in use since 2006. Side effects requiring immediate medical attention. Zostavax zoster vaccine is a one-dose high-potency live attenuated VZV vaccine that boosts VZV-specific CMI and this is its presumed mechanism of action.
Before sharing sensitive information make sure youre on a federal government site. This vaccine should not be used during pregnancy. For example you could use Zostavax if a person is allergic to Shingrix prefers Zostavax or requests immediate.
Along with its needed effects zoster vaccine live the active ingredient contained in Zostavax may cause some unwanted. Shingrix reduced the risk of shingles in 979 of patients 70 years and older while Zostavax protected against shingles in 41 of subjects aged 70 to 79 years. ZOSTAVAX is a live attenuated varicella-zoster vaccine and administration to individuals who are immunosuppressed or immunodeficient may result in disseminated varicella-zoster virus disease including fatal outcomes.
Zostavax zoster vaccine live reduced the risk of developing shingles by 51 in those 60 years years old. In 2016 the live attenuated zoster vaccine Zostavax Merck and Co USA was introduced into the Australian National Immunisation Program for people aged 70 years who are not significantly immunocompromised. Although Zostavax is licensed for use from age 50 years and is effective in this age group the burden of shingles disease is.
It can be administered concurrently with all other live and inactivated vaccines including those routinely recommended for people 60 years old and older such as influenza and pneumococcal vaccines. Some of the dosage forms listed on this page may not apply to the brand name Zostavax. When this virus becomes active again in an adult it can cause herpes zoster or shingles.
ZOSTAVAX cannot be used to treat existing shingles or the pain associated with existing shingles. ZOSTAVAX contains a weakened chickenpox virus varicella-zoster virus. Although the licensed HZ live-attenuated vaccine Zostavax has been shown to be safe and effective in older adults it is currently not recommended for use in patients with primary and acquired immunodeficiency because of its potential to trigger the development of HZ within 46 weeks after vaccination 15 30 4448.
Applies to zoster vaccine live. Zostavax is a FDA licensed vaccine that helps to reduce the risk of getting herpes zoster or shingles in individuals 50 years. It is not known if zoster vaccine passes into breast milk.
Thus it is important to administer more than 1 vaccine during a single visit. ZOSTAVAX is a vaccine used to prevent shingles zoster and zoster-related post-herpetic neuralgia PHN the long-lasting nerve pain that follows shingles. Zoster vaccine a live virus vaccine can be administered concurrently with all other live and inactivated vaccines including those routinely recommended for people 60 years of age or older such as influenza and pneumococcal vaccines.
Zostavax is a live attenuated vaccine formulated from the same varicella-zoster virus VZV strain Oka as the registered varicella chickenpox vaccine Varivax. Zostavax may still be used to prevent shingles in healthy adults 60 years and older. For more information see the Best Practices of the Advisory Committee on Immunization Practices ACIP.
During the clinical development of Zostavax which was mainly in the US the vaccine was. The vaccine should not be injected intramuscularly. Zoster Vaccine Live ZOSTAVAX A major impediment to administration of the portfolio of vaccines recommended for older adults is their low frequency of encounters with healthcare providers.
ZOSTAVAX is a vaccine that is used for adults 50 years of age or older to prevent shingles also known as zoster. The FDA and ACIP have routinely endorsed the safety and efficacy of. Zoster vaccine can be administered concurrently with all other live and inactivated vaccines including those routinely recommended for people 60 years of age or older such as influenza and pneumococcal vaccines says the.
Zostavax is indicated for the prevention of herpes zoster shingles and herpes zoster-related post-herpetic neuralgia in people aged 50 years of age or older. However it is not necessary to repeat vaccination if it is administered intramuscularly. People with a weakened immune system cannot have live vaccines.
If you administer Shingrix and another vaccine to someone on the same day give them at different anatomical sites eg different arms. Zostavax is a live virus vaccine. The gov means its official.
Product approval information for Zoster Vaccine also know as Zostavax. Zostavax zoster vaccine live is used to prevent herpes zoster virus shingles in people age 50 and older. Zostavax zoster vaccine live is administered subcutaneously as a single dose in the deltoid region.
It contains a weakened chickenpox virus varicella-zoster virus. Naturally occurring varicella-zoster infection is known to cause harm to the unborn baby. But Zostavax has higher potency on average at least 14 times greater than Varivax.
We report the administration of Zostavax in an immunocompromised patient with chronic lymph.

Why Is Merck Being Sued Over The Zostavax Vaccine Vb Attorneys

Shingles Vaccine Zostavax Is Causing What It S Designed To Prevent The Ring Of Fire Network

Zostavax Dosage Rx Info Uses Side Effects
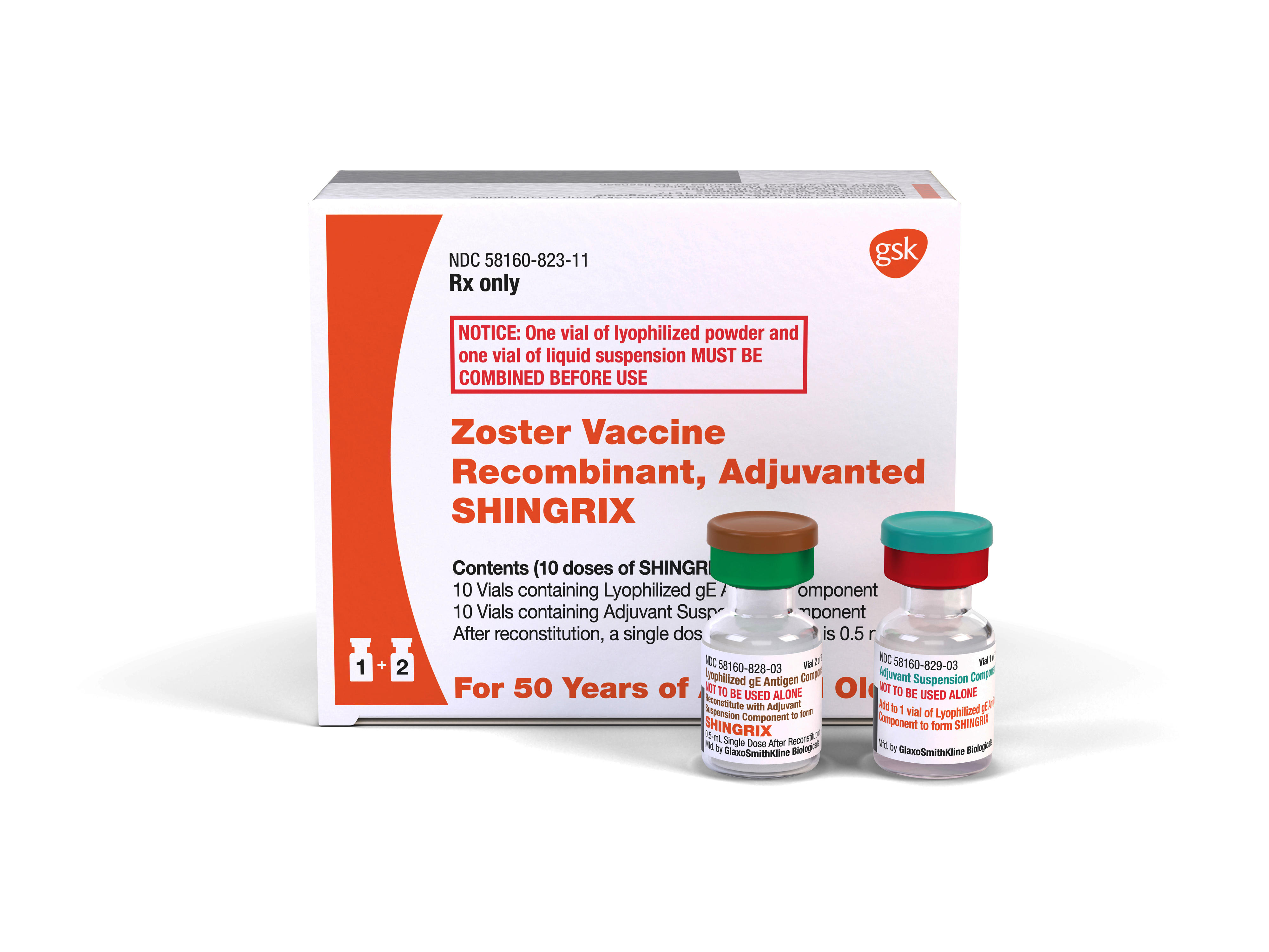
Who Should Get The New Shingles Vaccine Cbs News

Zostavax Shingles Vaccine Varicella Zoster Virus Vaccine Live Attenuated Injection Single Dose Vial

Zostavax Shingles Vaccine National Vaccine Support Group

Merck Shingles Vaccine Zostavax Lawsuit Lawyer Carlson Law Firm
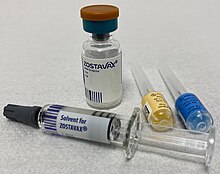
0 Response to "is zostavax a live vaccine"
Post a Comment